Resveratrol and Leukaemia - Integrative Cancer Network
Resveratrol kháng JTC26
From Wikipedia, the free encyclopedia
 | |
 | |

Chemical structures of cis- ((Z)-resveratrol, left) and trans-resveratrol ((E)-resveratrol, right)[1]
| |
| Names | |
|---|---|
| Other names
trans-3,5,4′-Trihydroxystilbene;
3,4′,5-Stilbenetriol; trans-Resveratrol; (E)-5-(p-Hydroxystyryl)resorcinol; (E)-5-(4-hydroxystyryl)benzene-1,3-diol | |
| Identifiers | |
3D model (JSmol)
| |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.121.386 |
| KEGG | |
PubChem CID
| |
| RTECS number | CZ8987000 |
| UNII | |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.25 g·mol−1 |
| Appearance | white powder with slight yellow cast |
| Melting point | 261 to 263 °C (502 to 505 °F; 534 to 536 K)[2] |
| Solubility in water | 0.03 g/L |
| Solubility in DMSO | 16 g/L |
| Solubility in ethanol | 50 g/L |
| UV-vis (λmax) | 304nm (trans-resveratrol, in water) 286nm (cis-resveratrol, in water)[1] |
| Hazards | |
| Safety data sheet | Fisher Scientific[2] Sigma Aldrich[3] |
| R-phrases(outdated) | R36 (irritating to eyes)[3] |
| S-phrases(outdated) | S26 (in case of contact with eyes, rinse immediately with plenty of water and seek medical advice)[3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
| 23.2 µM (5.29 g)[4] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Infobox references | |
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced by several plants in response to injury or, when the plant is under attack by pathogens such as bacteria or fungi.[5][6] Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries.[7]
Although it is used as a dietary supplement, there is no clear evidence that consuming resveratrol affects life expectancyor human health.[8][9]
Health effects[edit]
Heart disease[edit]
There is no evidence of benefit from resveratrol in those who already have heart disease.[10] A 2014 Chinese meta-analysis found weak evidence that high-dose resveratrol supplementation could reduce systolic blood pressure.[11]
Cancer[edit]
As of 2016, there is no evidence of an effect of resveratrol on cancer in humans.[12]
Metabolism[edit]
Lifespan[edit]
There is no evidence for an effect of resveratrol on lifespan in humans as of 2011.[15]
Adverse effects[edit]
In 2010, GlaxoSmithKline (GSK) suspended a small clinical trial of SRT501, a proprietary form of resveratrol, due to safety concerns, and terminated the study later that year.[16]
Although limited human studies have shown resveratrol is well-tolerated,[11][14] one clinical study of Alzheimer's diseasepatients showed there were side effects from daily intake of up to 2 grams, including nausea, diarrhea, and weight loss.[17]
Pharmacology[edit]
Pharmacodynamics[edit]
Although in vitro studies indicate resveratrol activates sirtuin 1[18] and PGC-1α, and affects functioning of mitochondria,[19]other research disputes this effect.[20][21]
In cells treated with resveratrol, an increase is observed in the action of MnSOD (SOD2)[22] and in GPER activity.[23]
Pharmacokinetics[edit]
One way of administering resveratrol in humans may be buccal delivery, that is without swallowing, by direct absorption through tissues on the inside of the mouth. When one milligram of resveratrol in 50 ml 50% alcohol/ water solution was retained in the mouth for one minute before swallowing, 37 ng/ml of free resveratrol was measured in plasma two minutes later. This level of unchanged resveratrol in blood can only be achieved with 250 mg of resveratrol taken in a pill form.[24] However, the viability of a buccal delivery method is called into question due to the low aqueous solubility of the molecule. For a drug to be absorbed transmucosally it must be in free-form or dissolved.[25][26] Resveratrol fits the criteria for oral transmucosal dosing, except for this caveat. The low aqueous solubility greatly limits the amount that can be absorbed through the buccal mucosa. Resveratrol that is attempted to be taken buccally was expected to pass through the mucous membrane of the mouth and be absorbed as an oral dose,[27] however, the need to explore buccal delivery in future pharmaceutical formulations was expressed.[26][28]
While 70% of orally administered resveratrol is absorbed, its oral bioavailability is approximately 0.5% due to extensive hepatic glucuronidation and sulfation.[29]Resveratrol given in a proprietary formulation SRT-501 (3 or 5 g), developed by Sirtris Pharmaceuticals, reached five to eight times higher blood levels. These levels did approach the concentration necessary to exert the effects shown in animal models and in vitro experiments.[30]
In rats, less than 5% of the oral dose was observed as free resveratrol in blood plasma.[31] There is a hypothesis that resveratrol from wine could have higher bioavailability than resveratrol from a pill.[32]
In a human study involving oral administration of 500 mg over 13 weeks, resveratrol was detected in cerebrospinal fluid, indicating that it had crossed the blood-brain barrier.[17]
Metabolism[edit]
Resveratrol gets extensively metabolized in the body, with the liver and lungs as the major sites of its metabolism.[33]
Chemistry[edit]
Resveratrol (3,5,4'-trihydroxystilbene) is a stilbenoid, a derivative of stilbene.
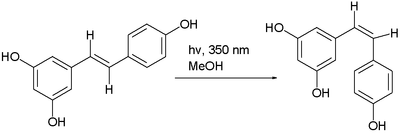
It exists as two geometric isomers: cis- (Z) and trans- (E), with the trans-isomer shown in the top image. The trans- and cis-resveratrol can be either free or bound to glucose.[34]
The trans- form can undergo isomerization to the cis- form when exposed to ultraviolet irradiation,[35] a process called photoisomerization:[36]
One study showed that ultraviolet irradiation to cis-resveratrol induces further photochemical reaction, producing a fluorescent molecule named "Resveratrone".[37]
Trans-resveratrol in the powder form was found to be stable under "accelerated stability" conditions of 75% humidity and 40 °C in the presence of air.[38] The transisomer is also stabilized by the presence of transport proteins.[39] Resveratrol content also was stable in the skins of grapes and pomace taken after fermentation and stored for a long period.[40] lH- and 13C-NMR data for the four most common forms of resveratrols are reported in literature.[34]
Biosynthesis[edit]
Resveratrol is produced in plants by the action of the enzyme, resveratrol synthase.[41]
Biotransformation[edit]
The grapevine fungal pathogen Botrytis cinerea is able to oxidise resveratrol into metabolites showing attenuated antifungal activities. Those include the resveratrol dimers restrytisol A, B, and C, resveratrol trans-dehydrodimer, leachinol F, and pallidol.[42] The soil bacterium Bacillus cereus can be used to transform resveratrol into piceid (resveratrol 3-O-beta-D-glucoside).[43]
Occurrences[edit]
Plants[edit]
Resveratrol is a phytoalexin, a class of compounds produced by many plants when they are infected by pathogens or physically harmed by cutting, crushing, or ultraviolet radiation.[44]
Plants that synthesize reservatrol include knotweeds, pine trees including Scots pine and Eastern white pine, grape vines, peanut plants, cocoa bushes, and Vacciniumshrubs that produce berries, including blueberries, raspberries, mulberries, cranberries, and bilberries.[5][7][44]
Foods[edit]
The levels of resveratrol found in food varies considerably, even in the same food from season to season and batch to batch.[5]
Wine and grape juice[edit]
| Beverage | Resveratrol (mean/range) (mg/150 ml)[45] |
|---|---|
| Red wine | 0.27 / 0 —2.78 |
| Rose wine | 0.12 / 5.00e-03—0.29 |
| White wine | 0.04 / 0.00—0.17 |
| Sparkling wine | 9.00e-03 / 8.00e-03 —1.00e-02 |
| Green grape juice | 5.08e-03 / 0.00 —1.00e-02 |
In a 2007 review of published resveratrol concentrations, the average in red wines is 1.9±1.7 mg trans-resveratrol/L (8.2±7.5 µM, ranging from nondetectable levels to 14.3 mg/l (62.7 μM) trans-resveratrol. Levels of cis-resveratrol follow the same trend as trans-resveratrol.[46]
In general, wines made from grapes of the Pinot Noir and St. Laurent varieties showed the highest level of trans-resveratrol, though no wine or region can yet be said to produce wines with significantly higher concentrations than any other wine or region.[46] Champagne and vinegar also contain appreciable levels of resveratrol.[45]
Red wine contains between 0.2 and 5.8 mg/l, depending on the grape variety. White wine has much less because red wine is fermented with the skins, allowing the wine to extract the resveratrol, whereas white wine is fermented after the skin has been removed.[5] The composition of wine is different from that of grapes since the extraction of resveratrol from grapes depends on the duration of the skin contact, and the resveratrol 3-glucosides are in part hydrolysed, yielding both trans- and cis-resveratrol.[5]
Selected foods[edit]
| Food | Serving | Total resveratrol (mg)[5] |
|---|---|---|
| Peanuts (raw) | 1 cup (146 grams) | 0.01 – 0.26 |
| Peanut butter | 1 cup (258 grams) | 0.04 – 0.13 |
| Red grapes | 1 cup (160 grams) | 0.24 – 1.25 |
| Cocoa powder | 1 cup (200 grams) | 0.28 – 0.46 |
Ounce for ounce, peanuts have about 25% as much resveratrol as red wine.[5] Peanuts, especially sprouted peanuts, have a content similar to grapes in a range of 2.3 to 4.5 μg/g before sprouting, and after sprouting, in a range of 11.7 to 25.7 μg/g, depending upon peanut cultivar.[44][45]
Mulberries (especially the skin) are a source of as much as 50 micrograms of resveratrol per gram dry weight.[47]
Dietary supplements[edit]
Harvard University scientist and professor David Sinclair co-founded Sirtris Pharmaceuticals, the initial product of which was a resveratrol formulation;[48] Sinclair became known for making statements about resveratrol like: “(It's) as close to a miraculous molecule as you can find.... One hundred years from now, people will maybe be taking these molecules on a daily basis to prevent heart disease, stroke, and cancer.”[49] Most of the anti-aging field was more cautious, especially with regard to what else resveratrol might do in the body and its lack of bioavailability.[49][50]
Sinclair is often quoted and pictured in online ads for resveratrol supplements, many of which implied endorsement of the advertised product even though Sinclair had not endorsed them.[51]
As a result of news coverage of Sinclair and others,[52][53] sales of supplements increased in 2006,[54] despite studies cautioning that benefits to humans are unproven.[54][55][56]
Supplements vary in purity and can contain anywhere from 50 percent to 99 percent resveratrol.[citation needed]
History[edit]
The first mention of resveratrol was in a Japanese article in 1939 by Michio Takaoka, who isolated it from Veratrum album, variety grandiflorum, and later, in 1963, from the roots of Japanese knotweed.[44][57][58][59]
Research[edit]
A 2011 systematic review of existing resveratrol research demonstrated there was not enough evidence to demonstrate its effect on longevity or human diseases, nor could there be recommendations for intake beyond the amount normally obtained through dietary sources, estimated as being less than 4 mg/day.[8] Much of the research showing positive effects has been done on animals, with insufficient clinical research on humans.[8] Resveratrol research in animals and humans remains active.[60][61]
Cancer[edit]
As of 2014, the results of studies on laboratory animals or human clinical trials concerning the effects of resveratrol on cancer are inconsistent,[12] even if massive doses of resveratrol are used.[62]
Neurological studies[edit]
A preliminary, one-year clinical trial of subjects with Alzheimer's disease showed that consuming 2 grams of resveratrol daily was well-tolerated and reduced some disease biomarkers in cerebrospinal fluid and blood, although other biomarkers and progressive dementia were unaffected.[17] Other preliminary human studies indicated that short-term ingestion of resveratrol increased cerebral blood flow in normal subjects[63] and in people with diabetes.[64] Resveratrol is under study for its potential to limit secondary damage after ischemic stroke or acute brain trauma.[65]
Cardiovascular studies[edit]
Although moderate drinking of red wine is generally associated with reduced risk of heart disease,[66] an association known as "the French paradox",[67] there is little evidence that resveratrol in red wine may have a role in this possible effect.[68]
Antidiabetic studies[edit]
Animal studies are being conducted to discern potential metabolic and antidiabetic effects of resveratrol.[69] In vitro, resveratrol was shown to act as an agonist of Peroxisome proliferator-activated receptor gamma, a nuclear receptor under pharmacological research as a potential treatment for type 2 diabetes.[70] Although one systematic review and meta-analysis noted that resveratrol is a "leading candidate" compound for serving as an adjunct pharmacotherapy for type 2 diabetes,[71] there is little evidence for its use as a possible treatment for diabetes.[69]
Skin[edit]
Despite considerable in vitro and animal research, there is no evidence that resveratrol taken orally or topically has any effect on human skin.[72] Preliminary studies have been conducted on resveratrol to understand its potential as a therapy for melanoma.[73][74]
Related compounds[edit]
- Dihydro-resveratrol
- Epsilon-viniferin, Pallidol and Quadrangularin A three different resveratrol dimers
- Trans-diptoindonesin B, a resveratrol trimer
- Hopeaphenol, a resveratrol tetramer
- Oxyresveratrol, the aglycone of mulberroside A, a compound found in Morus alba, the white mulberry[75]
- Piceatannol, an active metabolite of resveratrol found in red wine
- Piceid, a resveratrol glucoside
- Pterostilbene, a doubly methylated resveratrol
- 4'-Methoxy-(E)-resveratrol 3-O-rutinoside, a compound found in the stem bark of Boswellia dalzielii[76]

No comments:
Post a Comment