Malondialdehyde Production in Jurkat T Cells
Malondialdehyde kháng Jurkat
From Wikipedia, the free encyclopedia
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
propanedial
| |||
| Other names
Malonic aldehyde; Malonodialdehyde; Propanedial; 1,3-Propanedial ; Malonaldehyde
| |||
| Identifiers | |||
3D model (JSmol)
| |||
| Abbreviations | MDA | ||
| ChemSpider | |||
| KEGG | |||
PubChem CID
| |||
| Properties | |||
| C3H4O2 | |||
| Molar mass | 72.06 g·mol−1 | ||
| Appearance | Needle-like solid[1] | ||
| Density | 0.991 g/mL | ||
| Melting point | 72 °C (162 °F; 345 K) | ||
| Boiling point | 108 °C (226 °F; 381 K) | ||
| Hazards | |||
| US health exposure limits (NIOSH): | |||
PEL (Permissible)
| none[1] | ||
REL(Recommended)
| Ca[1] | ||
IDLH (Immediate danger)
| Ca [N.D.][1] | ||
| Related compounds | |||
Related alkenals
| Glucic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
| Infobox references | |||
Malondialdehyde (MDA) is the organic compound with the formula CH2(CHO)2. The structure of this species is more complex than this formula suggests. This reactive species occurs naturally and is a marker for oxidative stress.
Contents
[hide]Structure and synthesis[edit]
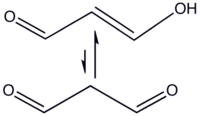
- CH2(CHO)2 → HOCH=CH-CHO
In organic solvents, the cis-isomer is favored, whereas in water the trans-isomer predominates.
Malondialdehyde is a highly reactive compound that is not typically observed in pure form. In the laboratory it can be generated in situ by hydrolysis of 1,1,3,3-tetramethoxypropane, which is commercially available.[2] It is easily deprotonated to give the sodium salt of the enolate (m.p. 245 °C).
Malondialdehyde results from lipid peroxidation of polyunsaturated fatty acids.[3] It is a prominent product in Thromboxane A2 synthesis wherein cyclooxygenase 1 or cycloxygenase 2 metabolizes arachidonic acid to prostaglandin H2 by platelets and a wide array of other cell types and tissues. This product is further metabolized by Thromboxane synthase to Thromboxane A2, 12-Hydroxyheptadecatrienoic acid, and malonyldialdehyde.[4][5] Alternatively, it may rearrange non-enzymatically to a mixture of 8-cis and 8-trans isomers of 12-hydroxyeicosaheptaenoic acid plus malonyldialdehyde (see 12-Hydroxyheptadecatrienoic acid).[6] The degree of lipid peroxidation can be estimated by the amount of malondialdehyde in tissues.[3]
Biochemistry[edit]
Reactive oxygen species degrade polyunsaturated lipids, forming malondialdehyde.[7] This compound is a reactive aldehyde and is one of the many reactive electrophile species that cause toxic stress in cells and form covalent protein adducts referred to as advanced lipoxidation end-products (ALE), in analogy to advanced glycation end-products (AGE).[8] The production of this aldehyde is used as a biomarker to measure the level of oxidative stressin an organism.[9][10]
Malondialdehyde reacts with deoxyadenosine and deoxyguanosine in DNA, forming DNA adducts, the primary one being M1G, which is mutagenic.[11] The guanidine group of arginine residues condense with malondialdehyde to give 2-aminopyrimidines.
Human ALDH1A1 aldehyde dehydrogenase is capable of oxidizing malondialdehyde.
Analysis[edit]
Malondialdehyde and other thiobarbituric reactive substances (TBARS) condense with two equivalents of thiobarbituric acid to give a fluorescent red derivative that can be assayed spectrophotometrically.[2][12] 1-Methyl-2-phenylindole is an alternative more selective reagent.[2]
Hazards and pathology[edit]
Malondialdehyde is reactive and potentially mutagenic.[13] It has been found in heated edible oils such as sunflower and palm oils.[14]
Corneas of patients suffering from keratoconus and bullous keratopathy have increased levels of malondialdehyde, according to one study.[15] MDA also can be found in tissue sections of joints from patients with osteoarthritis.[16]
No comments:
Post a Comment