[HTML] … effect of combined quercetin and recombinant adenoviral vector expressing human wild-type p53, GM-CSF and B7-1 genes on hepatocellular carcinoma cells …
Quercetin kháng Bel-7402
From Wikipedia, the free encyclopedia
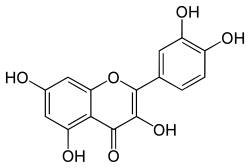 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˈkwɜːrsᵻtᵻn/ |
| IUPAC name
2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one
| |
| Other names
5,7,3′,4′-flavon-3-ol, Sophoretin, Meletin, Quercetine, Xanthaurine, Quercetol, Quercitin, Quertine, Flavin meletin
| |
| Identifiers | |
3D model (JSmol)
| |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.807 |
| KEGG | |
PubChem CID
| |
| UNII | |
| Properties | |
| C15H10O7 | |
| Molar mass | 302.236 g/mol |
| Appearance | yellow crystalline powder[1] |
| Density | 1.799 g/cm3 |
| Melting point | 316 °C (601 °F; 589 K) |
| Practically insoluble in water; soluble in aqueous alkaline solutions[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Infobox references | |
Quercetin is a plant polyphenol from the flavonoid group, found in many fruits, vegetables, leaves, and grains. It can be used as an ingredient in supplements, beverages, or foods.
Contents
[hide]Health effects[edit]
While quercetin supplements have been promoted for prevention and treatment of cancer, "there is no reliable clinical evidence that quercetin can prevent or treat cancer in humans".[2] Also, there is no conclusive evidence that consuming foods rich in quercetin reduces the risk of cancer or any other disease.[3]
Quercetin supplements have also been promoted for the treatment of a wide spectrum of other diseases.[4] However, the European Food Safety Authority evaluated possible health claims associated with consumption of quercetin, and found that no cause-and-effect relationship has been established for any physiological effect in human health or diseases.[5]
Drug interactions[edit]
Quercetin is contraindicated with some antibiotics; it may interact with fluoroquinolones (a class of antibiotics), as quercetin competitively binds to bacterial DNA gyrase. Whether this inhibits or enhances the effect of fluoroquinolones is not certain.[6]
As paclitaxel is metabolized primarily by CYP2C8, its bioavailability may be increased unpredictably by quercetin, potentially leading to harmful side effects.[7][8]
Occurrence[edit]
Quercetin is a flavonoid widely distributed in nature. The name has been used since 1857, and is derived from quercetum(oak forest), after Quercus.[9][10] It is a naturally occurring polar auxin transport inhibitor.[11]
Quercetin is one of the most abundant dietary flavonoids with an average daily consumption of 25–50 mgs.[12]
| Foods containing quercetin | Quercetin (mg/100g of edible portion) |
|---|---|
| capers, raw | 234[13] |
| capers, canned | 173[13] |
| lovage | 170[13] |
| dock like sorrel | 86[13] |
| radish leaves | 70[13] |
| carob fiber | 58[13] |
| dill | 55[14] (48-110)[15] |
| cilantro | 53[13] |
| Hungarian wax pepper | 51[13] |
| fennel leaves | 48.8[13] |
| onion, red | 32[16] |
| radicchio | 31.5[13] |
| watercress | 30[16] |
| buckwheat | 23[17] |
| kale | 23[16] |
| chokeberry | 19[16] |
| cranberry | 15[16] |
| lingonberry | 13[16] |
| plums, black | 12[16] |
| cow peas | 11[16] |
| sweet potato | 10[16] |
| blueberry, cultivated | 8[16] |
| sea buckthorn berry | 8[16] |
| rowanberry | 7[16] |
| crowberry | 5[16] |
| prickly pear cactus fruits | 5[16] |
| apples, Red Delicious | 4[16] |
| broccoli | 3[16] |
| bilberry | 3[16] |
| tea, black or green Camellia sinensis | 2[16] |
| red kidney beans, raw (powdered) | 0.0603 +/- 0.0307[18] |
In red onions, higher concentrations of quercetin occur in the outermost rings and in the part closest to the root, the latter being the part of the plant with the highest concentration.[19] One study found that organically grown tomatoes had 79% more quercetin than non-organically grown fruit.[20] Quercetin is present in various kinds of honey from different plant sources.[21]
Biosynthesis[edit]
In plants, phenylalanine is converted to 4-coumaroyl-CoA in a series of steps known as the general phenylpropanoid pathway using phenylalanine ammonia-lyase, cinnamate-4-hydroxylase, and 4-coumaroyl-CoA-ligase.[22] One molecule of 4-coumaroyl-CoA is added to three molecules of malonyl-CoA to form tetrahydroxychalcone using 7,2′-dihydroxy-4′-methoxyisoflavanol synthase. Tetrahydroxychalcone is then converted into naringenin using chalcone isomerase.
Naringenin is converted into eriodictyol using flavanoid 3′-hydroxylase. Eriodictyol is then converted into dihydroquercetin with flavanone 3-hydroxylase, which is then converted into quercetin using flavonol synthase.[22]
Glycosides[edit]
Quercetin is the aglycone form of a number of other flavonoid glycosides, such as rutin and quercitrin, found in citrus fruit, buckwheat and onions. Quercetin forms the glycosides quercitrin and rutin together with rhamnose and rutinose, respectively. Likewise guaijaverin is the 3-O-arabinoside, hyperoside is the 3-O-galactoside, isoquercitin is the 3-O-glucoside and spiraeoside is the 4′-O-glucoside. CTN-986 is a quercetin derivative found in cottonseeds and cottonseed oil. Miquelianin is the quercetin 3-O-β-D-glucuronopyranoside.[23]
Rutin degradation pathway[edit]
The enzyme quercitrinase can be found in Aspergillus flavus.[24] This enzyme hydrolyzes the glycoside quercitrin to release quercetin and L-rhamnose. It is an enzyme in the rutin catabolic pathway[25]
Pharmacology[edit]
Pharmacokinetics[edit]
The bioavailability of quercetin in humans is low and highly variable (0-50%), and is rapidly cleared with an elimination half-life of 1–2 hours after ingesting quercetin foods or supplements.[26] Following dietary ingestion, quercetin undergoes rapid and extensive metabolism that makes the biological effects presumed from in vitrostudies unlikely to apply in vivo.[27][28]
Metabolism[edit]
In rats, quercetin did not undergo any significant phase I metabolism.[29] In contrast, quercetin did undergo extensive phase II (conjugation) to produce metabolites that are more polar than the parent substance and hence are more rapidly excreted from the body. The meta-hydroxyl group of catechol is methylated by catechol-O-methyltransferase. Four of the five hydroxyl groups of quercetin are glucuronidated by UDP-glucuronosyltransferase. The exception is the 5-hydroxyl group of the flavonoid ring which generally does not undergo glucuronidation. The major metabolites of orally absorbed quercetin are quercetin-3-glucuronide, 3'-methylquercetin-3-glucuronide, and quercetin-3'-sulfate.[29][30]
In vitro pharmacology[edit]
Quercetin has been reported to inhibit the oxidation of other molecules and hence is classified as an antioxidant.[27][30] Quercetin contains a polyphenolic chemical substructure that stops oxidation by acting as a scavenger of free radicals that are responsible for oxidative chain reactions.[31]
Quercetin also activates or inhibits the activities of a number of proteins.[32] For example, quercetin is a non-specific protein kinase enzyme inhibitor.[27][30] Quercetin has also been reported to have estrogenic (female sex hormone like) activities by activating estrogen receptors. Quercetin activates both estrogen receptor alpha (ERα) and beta (ERβ)[33] with binding IC50s of 1015 nM and 113 nM respectively. Hence quercetin is somewhat ERβ selective (9 fold) and is roughly two to three orders of magnitude less potent than the endogenous estrogenic hormone 17β-estradiol.[34][35] In human breast cancer cell lines, quercetin has also been found to act as an agonist of the G protein-coupled estrogen receptor (GPER).[36][37]
Clinical research[edit]
Although quercetin is under basic and early-stage clinical research for a variety of disease conditions,[38][39] there is insufficient evidence on whether quercetin promotes DNA repair in humans.[5] Claims about the role of quercetin in liver and kidney system function, mental performance or cardiovascular health in the human body are insufficiently defined to draw any conclusions.[5] The US FDA has issued warning letters to emphasize that quercetin is not a defined nutrient nor an antioxidant, cannot be assigned a dietary content level, and is not regulated as a drug to treat any human disease.[40]